|
 |
 |
|
Research at the MoLNaC is geared towards the interface between
catalysis and material science.
Understanding (and possibly solving) chemical problems, especially of
industrial relevance, is our main activity. To this end we use the
armory of tools known as computational chemistry. The areas of
interest span from understanding structure and function relationship
in organometallic compounds, to unraveling the mechanistic of
catalysts at work, to soft condensed matter simulations. Finally, we
are not shy to tackle systems of biological interest. In all cases we
try to interact as much as possible with experimentalists. Often we
are unsatisfied with the available tools and/or models and we develop
new ones. Particular interest is devoted to development of methods for
modelling systems across time and length scales.
Following molecules rolling on the
potential energy surface from reactants to products is another game we play.
Here, there are three classes of reactions that we work on. a) stereospecific
olefin polymerizations.
We have investigated in great details almost all aspects of propene
polymerization by both heterogeneous and homogeneous catalytic systems. The
main contribution in the field certainly is the rationalization of the
mechanism of stereocontrol operative with these systems. Based on the original Corradini’s
mechanism, that rationalizes the formation of iso- and syndiotactic
polypropylene when C2 and CS-symmetric stereorigid
group 4 metallocenes are used (chiral-site stereocontol), we extended it to
rationalize the intriguing formation of syndiotactic polypropylene when a C2-symmetric
stereoflexible group 4 octahedral catalyst is used (chain-end stereocontrol),
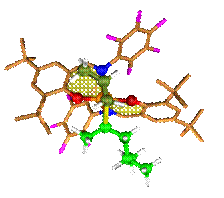
see Figure. Moreover, we also worked to rationalize the regiospecificity
exhibited by different systems, and we studied the catalyst structure/molecular
mass relationship exhibited by different systems.
Most favored
transition state for propene (dark green) secondary insertion into the
Ti-secondary chain (light green) for a bis(phenoxy-imine)Ti-based catalyst.
References
2) Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F.,
"Selectivity in Propene Polymerization with Metallocene Catalysts",
Chem. Rev., 2000, 100, 1253.
b) Mn-catalyzed
olefin epoxidations. The
actual mechanism operative with this class of catalysts is a very debated topic.
In this challenging field we benefited from a strong collaboration with Heiko
Jacobsen. We and other have shown that two electronic states are in competition,
and that some details of the actual reaction pathway depend on which electronic
state the system is. Nevertheless, we have been able to close the catalytic
cycle, including regeneration of the active species. Another contribution in
the field certainly is the mechanism of enantioselectivity we proposed.
c) The Nobel
2005 reaction, i.e. Ru and Mo-catalyzed olefin metathesis. In this field we have contributed the
first complete comparison of the phosphine and NHC-based Ru catalysts, i.e. of
the first and second generation Grubbs’ catalysts, trying to stress differences
in the steric and electronic properties of the two catalyst generations. Then,
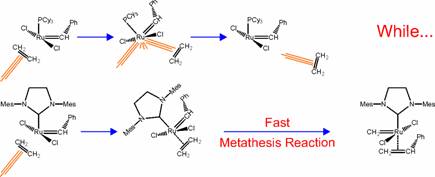
we have proposed a mechanism to rationalize the origin of enantioselectivity in
the Ru-based asymmetric olefin metathesis, see Figure. We are currently
extending this mechanism to the Mo-based Schrock’s catalysts.
Schematic
representation of the different reactivity of Grubbs’ first and second
generation catalysts.
|
|||